ポリソルベート80
ポリソルベート80(HX2)™
日油株式会社は、アルデヒド価や過酸化物価が極めて低い、高純度オレイン酸誘導体ポリソルベート80(HX2)™を開発しました。ポリソルベート80(HX2)™は、独自に開発・商業生産している純度99%以上の高純度オレイン酸(EXTRA OLEINE 99)を使用し、高度なエチレンオキサイド付加技術により不純物の生成を低レベルに抑え、更に、高度精製技術を駆使して不純物を徹底的に取り除いた画期的な高品質製品です。そのため、酸化されやすい高度不飽和脂肪酸や炭素数の異なる脂肪酸などの不純物をほとんど含んでいない、世界最高品質のポリソルベート80です。
この特徴は安全性にもつながっており、ラット肥満細胞および犬を用いたin vivo の試験においてもヒスタミン遊離が少ないことが確認されており、アレルギーを起こしにくいポリソルベート80として世界中から注目を集めています。
また、ポリソルベート80中の過酸化物によるタンパク製剤への影響に関する文献* において、モデルタンパク製剤(IL-2 mutein) は過酸化物価の低いポリソルベート80を用いた場合には変性しにくいのに対し、過酸化物価の高いポリソルベート80を用いた場合には著しく変性が進むことが示されています。そのため、過酸化物価が低く酸化安定性の高い日油株式会社のポリソルベート80(HX2)™を用いることで、製剤に必要な品質安定性を確保できるため、多くの製薬会社からの高い評価を受けています。
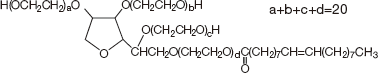

JP: Japanese Pharmacopoeia
EP: European Pharmacopoeia
USP/NF: National Formulary
ChP: Chinese Pharmacopoeia

Appearance comparison of Ultra-purity Polysorbate 80
Degranulation Test (An allergic study model)
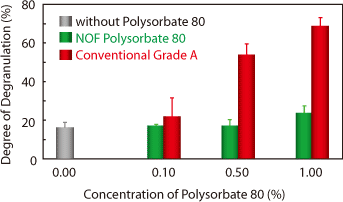
Effect of Polysorbate 80 on degranulation of RBL-2H3 mast cells. Cells were treated with different concentrations of Polysorbate 80 for 60 mins. The degree of degranulation was determined by measurement of the released ß-hexosaminidase into the supernatant.
Cell Toxicity Test
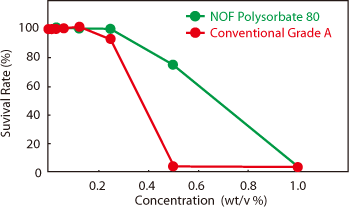
Effect of Polysorbate 80 concentration on the cytotoxicity using SIRC Cells. Cells were treated with each Polysorbate 80 for 24 hrs. The number of viable cells was determined by the Neutral Red Uptake method.
Influence on Basophillic leukocyte
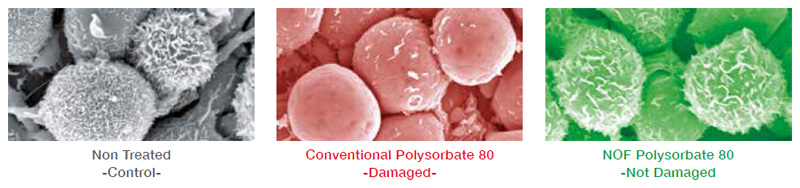
Basophilic leukocytes were immersed in 1% Polysorbate 80 solution for 30 min.
The cells were immobilized in glutaraldehyde and observed by SEM.
| Hemolysis Test | Survival Rate | Stability Test |
|---|---|---|
 |
 |
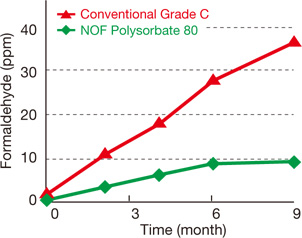 |
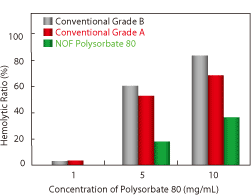
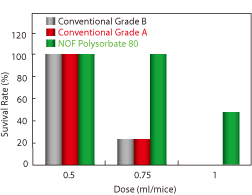
| Effect of Polysorbate 80 concentration on the Hemolytic Ratio. Red blood cells from the guinea pig were treated with Polysorbate 80 for 60 mins. The hemolytic ratio was determined by the absorbance of the solution at 576 nm. | Effect of Polysorbate 80 concentration on the Survival Rate of BALB/c mice. Polysorbate 80 was diluted with PBS and administered to mice by intravenous injection. | This formaldehyde test was performed under air sealed conditions at 40˚C. |
Specification
| Item | Specification | ||
|---|---|---|---|
| USP/NF Requirement | Identification | Meet the Requirement | |
| Viscosity(25°C, cSt) | 300-500 | ||
| Specific Gravity | 1.06-1.09 | ||
| Acid Value | NMT2.0 | ||
| Hydroxyl Value | 65-80 | ||
| Saponification Value | 45-55 | ||
| Water(%) | NMT3.0 | ||
| Residue on Ignition(%) | NMT0.25 | ||
| Residual Solvents | Meet the Requirement | ||
| Compoition of Fatty Acid(%) | Meet the Requirement | ||
| Ethylene Oxide and Dioxan(ppm) | NMT 1(Ethylene Oxide), NMT 10(Dioxan) | ||
| Peroxide Value | NMT10 | ||
| EP Requirement | Identification | Meet the Requirement | |
| Acid Value | NMT2.0 | ||
| Hydroxyl Value | 65-80 | ||
| Peroxide Value | NMT 10.0(Actual value 0-2) | ||
| Saponification Value | 45-55 | ||
| Composition of Fatty Acids(%) | Meet the Requirement | ||
| Ethylene Oxide and Dioxan(ppm) | NMT 1(Ethylene Oxide), NMT 10(Dioxan) | ||
| Water(%) | NMT3.0 | ||
| Total Ash(%) | NMT0.25 | ||
| JP Requirement | Appearence | Colorless or Brownish Yellow, Clear or Slightly Opalescent, Oily Liquid |
|
| Identification | Meet the Requirement | ||
| Composition of Fatty Acids(%) | Meet the Requirement | ||
| Acid Value | NMT2.0 | ||
| Saponification Value | 45-55 | ||
| Hydroxy Value | 65-80 | ||
| Purity(1) | Ethylene Oxide (ppm) | NMT1 | |
| 1,4-Dioxane (ppm) | NMT10 | ||
| Purity(2) Peroxide Value | NMT10.0 | ||
| Water(%) | NMT3.0 | ||
| Residue on Ignition(%) | NMT0.25 | ||
| ChP ”Polysorbate 80 (Ⅱ)” Requirement |
Description | Colorless or light yellow viscous liquid | |
| Relative Density | 1.06-1.09 | ||
| Viscosity (25°C,mm²/s) | 350-450 | ||
| Acid Value | NMT 1.0 | ||
| Saponification Value | 45-55 | ||
| Hydroxyl Value | 65-80 | ||
| Iodine Value | 18-24 | ||
| Peroxide Value | NMT 3 | ||
| Identification | Meet the Requirement | ||
| Acidity and Alkalinity | 5.0-7.5 | ||
| Absorbance | NMT 1.0 (A225) NMT 0.10 and no maximum absorption peak at 267 nm (A267) |
||
| Color | Not more intensely colored than reference solution Y2 | ||
| Freezing Test | It must not be frozen at ice bath for 24h | ||
| Water (%) | NMT 0.5 | ||
| Residue on Ignition (%) | NMT 0.1 | ||
| Heavy Metals (ppm) | NMT 10 | ||
| Arsenic (%) | NMT 0.0002 | ||
| Ethylene Oxide and Dioxane (%) | NMT 0.0001 (Ethylene Oxide), NMT 0.001 (Dioxane) |
||
| Ethylene Glycol, Diethylene Glycol and Triethylene Glycol (%) |
NMT 0.01 (Ethylene Glycol), NMT 0.01 (Diethylene Glycol), NMT 0.01 (Triethylene glycol) |
||
| Composition of Fatty Acids (%) | Meet the Requirement (NLT98%) | ||
| Bacterial Endotoxins (EU/mg) | LT 0.012 | ||
| Others | Color(APHA) | NMT160 | |
| Microbial Limit Test(cfu/ml) | NMT100 | ||
| Bacterial Endotoxins Test(EU/g) | NMT50 | ||
| Composition of Fatty Acid Oleic Acid Contents(%) | NLT99 | ||
Package Size : 1kg amber glass bottle, 10kg stainless steel can
The product is the grade of Polysorbate 80 in Chinese pharmacopoeia 2020.

